 |
case study | medical |
Harpoon Revolutionises Cancer Research Sixteen milliseconds – one-fifth the speed of the blink of an eye – can mean the difference between life and death for millions of people. How can such a miniscule amount of time have such a profound effect on so many? That’s about how long it takes for one infinitesimal cancer cell to adhere to a new location within the body. In as little as a day, a new tumor is born in a phenomenon known as metastasizing.
The American Cancer Society forecasts that nearly 1.5
million new cases of cancer will be diagnosed this year alone,
and for many patients, fear of metastasis will dominate their
treatment. It takes just one cell, measuring about one-fourth
the width of a human hair, to begin a new tumor in a secondary
site. Often renegade cells travel through the lymphatic
system, where they might get caught up in lymph nodes near the
primary site. Other times, they travel through the blood
stream, where they can make their way to any location within
the body. A Sticky Situation
Meghan Hoskins, a Ph.D. candidate in the
Bioengineering program at Penn State, under the advisement of
Robert Kunz, Ph.D. and Cheng Dong, Ph.D., is examining how
cancer cells stick to white blood cells, the defenders of the
blood stream, and how the flow of blood affects this adhesion.
Her work, funded by the National Cancer Institute and the PSU
Applied Research Laboratory, is based on the theory that, as
cancer cells travel through the blood stream, they are
attracted to areas where white blood cells are at work
fighting inflammation. This frightening concept, that cancer cells can actually use our own immune system against us, is the foundation of Hoskins research. Her goal is to accurately simulate previous experimental conditions of this phenomenon to validate her model, so that it may be used to further study the metastasis process. To do so, Hoskins is developing a simulated system, based on an existing rectangular test chamber in Professor Dong’s lab, designed to study the flow of these proteins to the white blood cells and how this affects the adhesion. A Model Approach
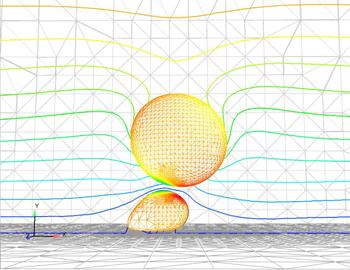
Existing experimental data suggests that
shear rate, the change in flow velocity within the micro
capillaries, can affect the adhesion of tumor cells. By
devising computational fluid dynamics models of the chamber,
Hoskins is calculating velocity profiles throughout the test
chamber and attempting to characterize the dynamic forces and
biochemistry at work during in vitro cell adhesion.
“Harpoon has been pretty important in my work because
I’m doing such small time steps with so much going on
simultaneously,” Hoskins says. “I need something that can work
quickly, and Harpoon has been very fast. Each time I make a
new grid, it takes less than 30 seconds. Without Harpoon, I
would have to generate each grid by hand, which could take
hours, depending on the complexity of the grid.” The results are exported to the AcuSolve flow solver for CFD analysis. Motion is then calculated by solving the six-degrees-of-freedom (6DOF) dynamics system for the cells in a Python script. This calculation allows Hoskins to determine exactly where and how fast the cells move at each step within the three-dimensional field. From 1 year to 1 dayEach simulation took approximately 2000 time steps with Harpoon running at each iteration. When a cell is deformable, Harpoon runs twice for each time step, so 4000 times per simulation. Hoskins estimates that she has run Harpoon approximately 60,000 times. Meshing this by hand would have taken over 21 years, using Harpoon it only took 21 days! Blood is Thicker than WaterSo far, Hoskins plans to model two experimental setups. The first is called a migration chamber – a rectangular flow chamber with holes in the bottom surface on which a filter is placed that allows cells to migrate through it. Endothelium cells, like those that form the inside lining of the blood vessels, are cultured on top of the filter. A solution of white blood cells and cancer cells are perfused through the inside. In this model, when only cancer cells are present in the chamber, there is significantly less migration of those cells through the endothelium than when white blood cells are also present. This suggests that the white blood cells influence the migration. In the second model, the chamber is sealed. Researchers can watch as the cells interact, collide and adhere to one another, and measure how much of this activity takes place. In this instance, it has been found that shear rate, or velocity, affects the cancer cell’s adhesion to white blood cells. But, the adhesion of white blood cells to the endothelial cells is affected by both shear rate and shear stress, or the force produced by the flow. Where the Model Meets Medicine
Hoskins’ mission is to understand why and
how the migration of cancer cells is affected by the fluid
dynamics of the system. This knowledge could help determine
targets for future therapeutic research. For example, if she
can identify that cancer-produced proteins carried through the
blood stream do significantly activate the white blood cells,
perhaps medical researchers can devise a way to block the
activity of those proteins.
|
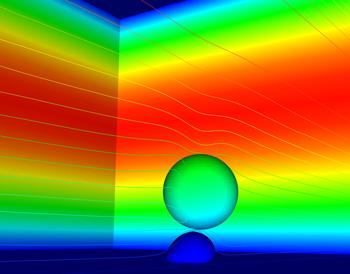
One deformable cancer cell traverses the white blood cell in this EnSight image. A cancer cell flows over the white blood cell without any bond interactions or deformation.
|